Designation
Extremity Drape
Description
Extremity drape set available for selection between different materials & standard size and fenestration.
Usage
EASY DRAPE Extremity Drapes are used to reduce risk of infection and create a focus on operation area in general surgical operations. Extremity drapes are specially designed for surgeries on extremities of body.
Packing
All components are packed in operational order so that efficiency is ensured. Depending on the preference 2 options are available:
- Pouch Pack: Drape is packed in a sterilization pouch with size depending on the selection of dimension and type of folding requested. Pouch has necessary peeling opening available.
- Blister Pack: Drape is packed inside a blister pack. Blister pack has a peeling side marked.
In both packing methods, packages show the information below:
- o Product series name & product name o Set type (dimensions and type of drape) o Set contents with quantities o Manufacturing date
- o Date of expiry o Lot number o Reference number
- o Sterilization indicator o Method of sterilization o Warnings (don’t use open packages, for single use only, latex free) o Manufacturer contact & company information
Sterility
Extremity Drapes (Ref: 10110508 – 10110510) are sterilized with EO sterilizer in house. Sterilization is documented and monitored through necessary forms, biological indicators, chemical indicators, dosimeters and data loggers.
It is possible to have NON-STERILE products upon request with same variety of selections from sizes and materials available. (Ref: 10120508 – 10120510)
Shelf Life
Product has been tested for stability. Test reports show that Extremity Drapes have a shelf life of 3 years.
Quality Certification & Compliance
All process across SUBMED is run in compliance with EN ISO 9001 & EN ISO 13485.
Independent third party laboratories tested EASY DRAPE series for packing sealing strength is in compliance with EN ISO 868-5, stability according to ASTM F 1980 and package leak tests according to ASTM F 1929. Thermoforming line is validated for temperature and pressure settings according to EN ISO 17025.
Sterilization process has been validated according to EN ISO 11135-1, for sterilization by Ethylene Oxide. Sterilizer is also validated by IQ and OQ tests.
Our manufacturing plant and clean room is in compliance and been tested for EN ISO 11605, EN ISO 14644-1, EN ISO 14644-2, EN ISO 14698-1 and EN ISO 14698-2 tests for bio contamination levels in order to assure relevant safety levels in all products.
EASY DRAPE series are tested for bio-burden according to EN ISO 11737-1. To achieve highest quality EO residue is also tested according to EN ISO 10993-7.
EASY DRAPE series are manufactured in compliance with EN 13795:2011. According to EN 13795:2011, EASY DRAPE series comply with EN ISO 22610:2006, EN ISO 11737-1:2006 + AC:2009, EN ISO 22612:2005, EN ISO 9073-10:2003, , EN 20811:1992, EN 13938:1999 and EN 29073-3:1992.
Extremity Drapes are CE Class IS products and have been certified by independent third parties from EU for compliance with standards.
Content
| Description | Qty. |
| Extremity Drape | 1 |
Materials of Selection
Extremity Drapes are packed in sterile packages. All Extremity Drapes can be made of from one of the below listed selections:
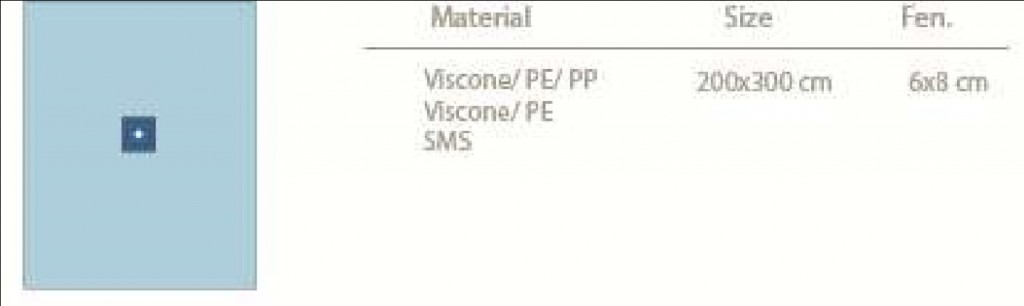
o Viscone – PE – PP (Obsolete) o Viscone – PE (54 gr/m2) o SMS (43 gr/m2)
Available Dimensions
Extremity Drapes are available in 200 x 300 cm dimension as standard. It is possible to have different dimensions for certain quantities upon request.
Extremity Drapes are prepared with standard size of fenestration 6 x 8 cm. It is possible to have a different fenestration opening size upon request.
Please request information from your Agent / Sales Representative for getting more information on tailor made solutions of EASY DRAPE series.
Technical Drawing
Storage
Store the product under normal conditions for healthcare material, in a cool and dry place protected from light. The use of medical grade paper and film guarantees its sterility for the duration of its shelf life under normal transport and storage conditions.
Comments
The product must be used by specialised personnel only. Check the package’s integrity before use. Do not use if the package is open or damaged.